-
Orthopedics/Sports
Real-world evidence study of regenerative medicine and shoulder surgery
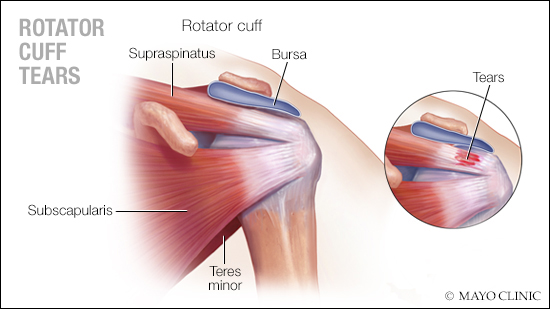
Applying regenerative medicine to a common shoulder surgery could have an impact on the need for follow-up revision surgery in some patients, according to a Mayo Clinic study of real-world evidence.
Mayo Clinic researchers analyzed the largest set of data available to determine if adding bone marrow aspirate concentrate to repaired tissue after standard rotator cuff surgery would improve outcomes for patients. Bone marrow aspirate is fluid taken from a patient's bone marrow that contains concentrated growth factors, stem cells and other specialized cells that may regenerate tissue and cartilage.
The analysis identified 760 patients who had a regenerative intervention added to augment rotator cuff repair surgery. Those patients were compared to 3,888 patients who did not have any biologic intervention at the time of surgery. The data indicated that 114 patients who opted for bone marrow aspirate concentrate at the time of surgery were less likely to need a second surgery.
The results of the Mayo Clinic study are published in the Orthopaedic Journal of Sports Medicine.
"The data we analyzed suggested a nearly threefold reduction in revision surgery in patients who received bone marrow aspirate concentrate, compared to those who did not," says Bradley Schoch, M.D., an orthopedic surgeon and principal investigator. "This procedure is growing in use throughout the practice of orthopedic surgery and commonly added as a surgical adjunct to rotator cuff tears."
A rotator cuff tear — the separation of tendons from the shoulder joint — is a leading cause of pain and disability for millions of people in the U.S. Arthroscopic surgery is the standard of care for repairing symptomatic rotator cuff tears. However, depending on the size of the tear and quality of the tendon, this surgery can fail, sometimes requiring revision surgery.
Regenerative medicine is an emerging field that is seeking new biotherapeutics to restore damaged cells, tissues and organs. One area of focus is on biologics that use sources from the human body — cells, blood, enzymes, tissues, genes or genetically engineered cells — for use in medicines. Mayo Clinic's Center for Regenerative Biotherapeutics is at the forefront of this movement and supports this study as part of its objective of delivering new regenerative biotherapeutics to the practice.
Studying real-world evidence
Preclinical research suggests potential benefits of orthobiologics, such as bone marrow aspirate concentrate, for healing damaged tendons. A few clinical trials have been conducted to try to establish scientific evidence of healing in humans. However, insurance considers orthobiologics to be experimental and does not reimburse for these procedures.
To accelerate Food and Drug Administration (FDA) approval of promising therapies, Congress passed the 21st Century Act in 2016 that allows data from real-world evidence to support regulatory decision-making. The FDA may now consider data from sources other than clinical trials, such as risks and benefits documented in electronic health records, lab reports and billing activities. In this Mayo Clinic study, researchers examined data from large insurance billing codes.
"The primary purpose of this study was to utilize a national administrative database to determine the association of bone marrow aspirate concentrate applied at the time of rotator cuff repair on revision surgery rates, compared to matched controls. It offers additional support to randomize control trials that often take years to complete and often are not definitive," says Shane Shapiro, M.D., an orthopedist and medical director at Mayo Clinic's Regenerative Medicine Therapeutic Suites in Florida. "This type of study allows stakeholders and health insurance companies to assess the risks, benefits and economic value of emerging medical interventions."
"Our results suggest patients undergoing rotator cuff repair surgery may benefit from the addition of bone marrow aspirate cells to surgically repair tissue," says Christopher Camp, M.D., a Mayo Clinic orthopedic surgeon and co-author of the study. "However, the exact therapeutic mechanisms of its action are still somewhat unclear."
The study team also analyzed the results of 646 patients who chose platelet-rich plasma at the time of rotator cuff repair surgery and found no measurable difference, compared to patients with no regenerative interventions. Platelet-rich plasma, also known as PRP, is an orthobiologic procedure in which platelets containing growth factors and healing potential are spun from blood and given back to the patient at the point of injury. Platelet-rich plasma has shown potential to ease other conditions, such as knee arthritis.
Clinical trials may offer more robust documentation of the benefits of orthobiologics for rotator cuff injuries. Higher-level clinical studies focusing on surgical factors will be needed to confirm whether bone marrow aspirate concentrate can prevent the need for a second rotator cuff repair surgery.
Related Articles







